Amcel research
Redox economic synthesis
In turn, the coupling of electrochemical synthesis with photochemical energy conversion provides a highly attractive approach to production of (fine) chemicals, that is sustainable in consumption of both energy and feedstocks. Furthermore, coupling electrochemically produced H2 directly with catalytic chemical transformations within one and the same electrolyzer is expected to offer entirely new possibilities to produce products that are otherwise very difficult (energy consuming, inefficient) to prepare.
Amcel aims in particular at improving the redox economy of chemical syntheses and the competitiveness of chemical industries employing these more environmentally friendly methods. This can be achieved with state-of-the art electrosynthetic approaches, by combining catalysis with electrochemistry. As such, it couples selectivity to energy efficiency! Many catalytic approaches can be combined with electrosynthesis, e.g. hydrogenation reactions, carbon-carbon and carbon-element bond formation, or complex skeletal rearrangements, which provide powerful methods to improve the selectivity of challenging transformations, including stereoselectivity as required for advanced pharmaceuticals.
“Redox economy refers to the endeavours to reduce the number of non-strategic (...) or corrective oxidation and reduction steps in synthesis, not only because these steps lower the overall efficiency of a synthesis, but also since many redox reactions are difficult to scale up in industrial settings and are frequently the source of noxious by-products and environmental problems”.Baran & Hoffmann, 2009. Angew. Chem. Int. Ed., 48, 2854.
Amcel Research Topics
It is evident that there is not a single strategy for the implementation of redox economic synthesis through the combination of synthesis, catalysis and electrochemistry. The electrification of the chemical industry has many aspects. The following research areas all carry the potential to establish Amcel as a world player in this field:
-
1. Homogeneous catalysis for water splitting and CO2 reduction
This involves:
- Hydrogen production through water electrolysis (the so-called ‘green hydrogen). This is strategically important as a sustainable alternative to the current production of hydrogen. Combining heterogeneous electrodes of electrolyzers with homogeneous catalysis and catalytic electrodes provides tremendous (yet unexplored) opportunities. Hydrogen production can also be combined with electrochemical oxidation processes for the chemical industry.
- The electrochemical conversion of CO2 to C1-C6 products (e.g. formic acid, oxalic acid, but also methanol or ethylene and other light olefins) is increasingly investigated as a way to exploit renewable electricity for the utilization of carbon dioxide emitted in concentrated streams (e.g. from cement or steel industry). To expand the scope of the electrochemical conversion of CO2 it is crucial to develop suitable electrocatalysts to enable the selective production of other, more challenging products, such as oxalic acid, methanol and ethylene.
-
2. Cogeneration of electricity and useful chemicals / paired electrolysis
Traditional fuel cells produce electricity from fuels such as H2 or MeOH, generating waste products of low/no value (CO2 or H2O). In novel organometallic fuel cells the production of electricity from other fuels can be coupled to the production of useful chemical products. This is attractive as it would allow using the energy that is typically dissipated as heat to generate electricity, thus making the process more sustainable and at the same time economically more attractive.
In principle, any redox reaction that is thermodynamically favourable lends itself to be carried out in an electrochemical cell. To make this possible it is crucial to develop suitable electrocatalysts, to achieve high selectivity towards the target product. Organometallic fuels cells are particularly promising to advance this field. Paired electrolysis is a similar approach, in which useful products are generated at both the anode and the cathode in an electrolyzer (in contrast to traditional electrosynthesis focusing on a single product), therefore maximizing the productive use of electricity and minimizing waste generation and energy consumption.
-
3. Waste and biomass as feedstock
A sustainable future relies on the use of waste and renewables (biomass) as feedstock. One of the key challenges to build part of future chemical processes and products on biomass-derived materials, especially towards specialty and fine chemicals, is selective deoxygenation. In particular arene C‒O bonds are extremely hard to cleave while e.g. selective deoxygenation of C‒O moieties in carbohydrates poses another major challenge.
The aim is to achieve mild and selective C‒O bond cleavage via sustainable redox-based processes. Waste streams (e.g. urea, phosphate, plastic soup) also provide tremendous opportunities to produce useful chemicals via electrochemical processes. Examples are urea, struvite, plastic waste, and CO2, e.g. electrocatalytic depolymerisation of plastics into value-added commodities that can be used as a feedstock for the production of fuels and polymers.
-
4. Electrocatalytic C‒N, C‒O and C‒C bond formation
Many stoichiometric and catalytic processes rely on the use of high-energy redox agents, which are in fact “over-oxidized” or “over-reduced”. Production of these redox agents is polluting and energy-intensive, and their application is associated with substantial waste formation, serious safety issues and unwanted energy losses. For example, the atom economy of catalytic nitrene transfer reactions, which have advanced as a powerful method for the amination of unactivated C–H bonds, is already much improved over ‘traditional’ stoichiometric amination reactions, but still relies on “over-oxidation” of the nitrogen source. This leads to waste formation and is associated with substantial energy losses.
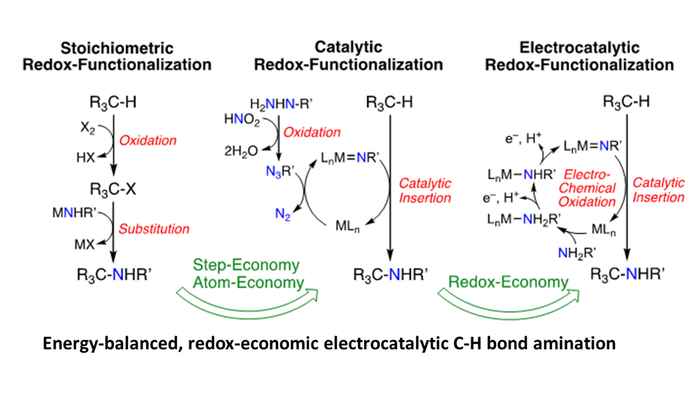
Nitrene transfer reactions are merely illustrative examples of the many possibilities offered by combining electrosynthesis and catalysis. Similar redox economy arguments hold for reactions involving highly reactive carbene or oxo species, which are highly useful for C‒O and C‒C bond formation. The goal is to develop efficient electrochemical coupling procedures, and catalytic C‒C, C‒N and C‒O bond formation reactions are the structure building class of transformations par excellence for chemistry, polymers, and organic materials. Direct redox catalytic processes need to be developed to replace key steps in current base and fine chemical manufacturing. The main aim is to deliver sustainable redox economic (i.e. step-, atom- AND energy-efficient) catalyzed electrosynthetic approaches to drive such overall thermodynamic “uphill” reactions (in absence of electrochemical energy input) in a clean and sustainable manner.
-
5. Materials for Electrochemistry
Emerging (photo)electrochemical applications put strong requirements on materials, e.g. for electrodes, membranes, porous separators and gas-diffusion materials, in terms of controlled mass transport, reduction of crossover, conversion rates and integration of different materials. This research also includes design, modeling and synthesis of new porous electrode materials that e.g. simultaneously act as electrode and capture chemicals (such as CO2). The materials used in electrochemical processes have to be earth‐abundant or at least made extremely efficient if rare materials are used. This requires new design principles and new experimental tools for materials synthesis and characterization. Understanding the degradation of materials, materials compatibility and materials interface/interphase is required in order to achieve developments beyond existing technologies.
-
6. Cell design/Electrochemical engineering
This research area aims to improve cell and stack design for particular application areas through increasing our understanding of the processes that take place at cell level. This includes innovative electrochemical flow cells, novel (organometallic) fuels cells and redox flow batteries and development of operando spectroscopic and spectrometric monitoring techniques close to electrodes.
-
7. Redox economic energy storage
Energy storage is key for a smooth transition to sustainable energy sources. In Amcel the following technologies are considered to be most promising: Fuel cells, redox flow batteries, structural batteries, hydrogen storage materials and chemical energy storage in molecules other than dihydrogen.